Submitting/Reporting
Reporting to the PMP Clearinghouse must be transmitted/submitted not more than 24 hours after the date on which the controlled substance is dispensed or by the end of the next business day. If you have issues or concerns that this document does not specifically address-a 24 hour helpline is available at 844-446-4767.
Manual Entry is an option for data submitters to enter their prescription information into the PMP Clearinghouse system using a form derived from the Universal Claims Form. It allows the entry of patient, prescriber, dispenser, and prescription information.
- The headings in BOLD ITALIC UNDERLINED are the headings for the different sections you will see when you are in a new UCF submission form. The bullet points under them are the items REQUIRED to be submitted, as well as the actual information that should be placed into each field.
- Use the following web address and log into your PMP Clearinghouse account: pmpclearinghouse.net
- Once logged in, select “UCF Submissions” at the top of the screen.
- Just below the “UCF Submissions” tab when the new page loads is a blue “New Claim Form” button. Click this button to begin the PMP form.
- Using drop down, select Indiana, Wait for the page to “reload” Patient.
- Select “Patient Animal” checkbox
- First Name – PAT08 = Owner’s First Name
Last Name – PAT07= Owner’s Last Name
Animal Name – PAT23 = Animal name species (ex: Fluffy canine)
Date of Birth = Owner DOB
Patient ID – no longer need to enter this info
ONLY NEEDS TO BE FILLED OUT IF THE PERSON LISTED ABOVE IS PICKING UP THE MEDICATIONS. IF SOMEONE OTHER THAN THE PERSON LISTED ABOVE IS PICKING UP THE MEDICATIONS, SKIP TO PATIENT ADDRESS (IF THIS SECTION IS SKIPPED, YOU MUST ENTER INFORMATION UNDER PICKUP/DROPOFF PERSON BELOW)
- Identity Type – PAT02 = use drop down to select one: Driver’s License, Military ID, Passport ID, State License Number
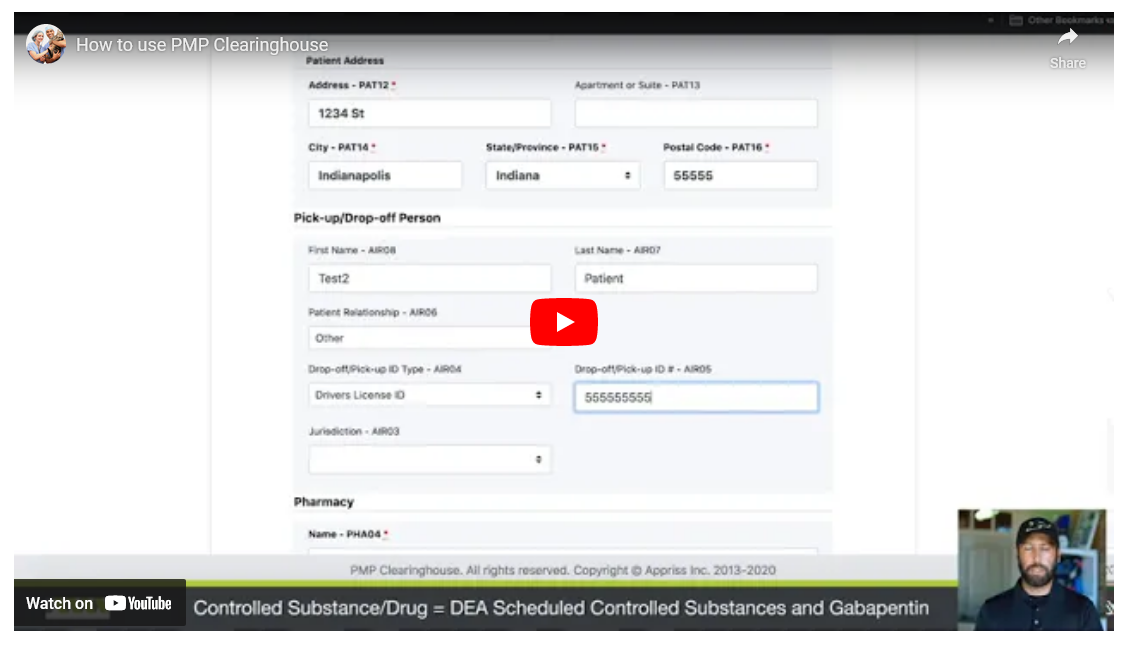
Identity Value- PAT03 = Identifying number from the ID (DL#, passport#, etc) - Patient Address: Address – PAT12 = Use the address of the person listed as the owner above.
- City – PAT14 = Use the city address of the person listed as the owner above.
- State/Province – PAT15 = Use the state address of the person listed as the owner above.
- Postal Code – PAT16 = Use the postal code of the address of the person listed as the owner above.
- Pick Up/Drop Off Person:
IF SOMEONE OTHER THAN THE OWNER LISTED ABOVE IS PICKING UP THE MEDICATION, THE FOLLOWING INFORMATION MUST BE FILLED OUT
- First Name- AIR08 = First name of Person physically picking up the meds
- Last Name- AIR 07 = Last name of the person physically picking up the meds
- Drop-Off/Pick-Up ID Type – AIR04 = Use dropdown menu to select the type of ID being used to enter this information
- Drop-Off/Pick-Up ID # – AIR05 = The identifier number on the ID being used to enter this information
Indiana State law requires an ID to pick up a controlled substance. - Pharmacies and other dispensers must collect an ID and record the identification number from anyone picking up a controlled substance.
- Records pertaining to controlled substances must be kept for 2 years. You can read about this in the federal code (link below).
- All information about what dispensers need to collect and report to the PDMP can be found in the INSPECT statute: IC 25-26-24
IC 25-26-24-17Controlled substance prescription monitoring program; information; prescription forms; identification Sec. 8.1. (a)
IC 25-26-24-6 (1)(E)”Identification number” (this section specifically addresses what to do if the patient is an animal) - Here is the link to the federal code on record keeping and inventory for controlled substances: 21 CFR 1304.04 (a)Maintenance of records and inventories
Pharmacy
When filling out the pharmacy information, first enter the DEA number as indicated below and then click the magnifying glass next to the form field. Most of the time the remaining information in this section will be auto-populated for the form. Sometimes though (especially the first time you do it), you may have to manually enter the information
- Name – PHA04 = DVM that orders the controlled substances for the hospital (typically the medical director)
- DEA Number – PHA03 = Clinic DEA Holder License number (typically the medical director) (Enter this number before entering in any other information in this section, then click the magnifying glass- usually will autopopulate remaining information)
- Address – PHA05 = Clinic Address (Address associated with the DEA number used above)
- City – PHA07 = Clinic City (associated with the DEA number used above)
- State – PHA08= Clinic State (associated with the DEA number used above)
- Postal Code – PHA09 = Clinic Zip code (associated with the DEA number used above)
Prescriber
- DEA Number – PRE02 = Prescribing DVM DEA Number (This is the DEA number of the DVM who prescribed the drug.) after entering, click the magnifying glass next to the form field and will usually autopopulate the remaining information in this category (usually have to enter rest of information manually the first time, but then will autopopulate thereafter)
- First Name – PRE06 = Prescribing DVM First Name
Last Name – PRE05 = Prescribing DVM Last name
Prescriptions
- Rx Number – DSP02 = Prescription number found on the Rx bottle being sent home
Refill Number – DSP06 = If this is the first time filled, this is 0. If it is the first time being refilled, this is 1, etc.- THIS IS NOT HOW MANY REFILLS ARE AUTHORIZED - Authorized Refills – DSP04 – number of refills allowed
- Days’ Supply – DSP10 = how many days the medication prescribed should last
- Date Written – DSP03 = Date the DVM ordered the Rx be filled
- Date Filled – DSP05 = Date the medication is sent home/out of the hospital/picked up
- Payment Type – DSP16 = Select the option “Patient Paid”
Drug Information
- The National Drug Code (NDC), as defined by the FDA, is “Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs.” The Rx number is assigned to a prescription by the dispensing clinic/pharmacy. IF IT IS A COMPOUNDED DRUG, CALL THE COMPOUNDING PHARMACY TO OBTAIN THE NDC NUMBER.
- – When reporting NDC numbers to the clearinghouse, they must be in a (5)-(4)-(2) format. This means, that the 3-segment number previously described must have 5 digits in the first section, 4 digits in the second section, and 2 digits in the last section. The NDC number can be found printed directly on the bottle of the medication. If any of the 3 sections indicated have less digits than the format requires, you must put a “0” before the number. An example is Gabapentin 100mg Capsules which has an NDC number of 49483-605-50. As we see, the second section only has 3 numbers, and reporting requires it to have 4 numbers. Therefore, when going to report this drug, it would be reported as 49483-0605-50. Entering the “0” before the “605” gives the second section a 4-digit code as required.
- NDC Number – DSP08 = NDC Number of the medication listed in the 5-4-2 format. If the NDC number is less than the 5 digits, 4 digits, or 2 digits, add a zero to the front of the number. (i/e if a drug NDC is 1234-123-1, it should be entered as 01234-0123-01 ENTER THE NDC NUMBER WITHOUT HYPHENS.
- Quantity – DSP09 = quantity of product filled
- Units – DSP11 = drop down and select one: each, milligram, milliliter
- Select the “SAVE” button- follow prompts on next page to confirm submission
When filling out the pharmacy information, first enter the DEA number as indicated below and then click the magnifying glass next to the form field. Most of the time the remaining information in this section will be auto-populated for the form. Sometimes though (especially the first time you do it), you may have to manually enter the information
- Name – PHA04 = DVM that orders the controlled substances for the hospital (typically the medical director)
- DEA Number – PHA03 = Clinic DEA Holder License number (typically the medical director) (Enter this number before entering in any other information in this section, then click the magnifying glass- usually will autopopulate remaining information)
- Address – PHA05 = Clinic Address (Address associated with the DEA number used above)
- City – PHA07 = Clinic City (associated with the DEA number used above)
- State – PHA08= Clinic State (associated with the DEA number used above)
- Postal Code – PHA09 = Clinic Zip code (associated with the DEA number used above)
Prescriber
- DEA Number – PRE02 = Prescribing DVM DEA Number (This is the DEA number of the DVM who prescribed the drug.) after entering, click the magnifying glass next to the form field and will usually autopopulate the remaining information in this category (usually have to enter rest of information manually the first time, but then will autopopulate thereafter)
- First Name – PRE06 = Prescribing DVM First Name
Last Name – PRE05 = Prescribing DVM Last name
Prescriptions
- Rx Number – DSP02 = Prescription number found on the Rx bottle being sent home
Refill Number – DSP06 = If this is the first time filled, this is 0. If it is the first time being refilled, this is 1, etc.- THIS IS NOT HOW MANY REFILLS ARE AUTHORIZED - Authorized Refills – DSP04 – number of refills allowed
- Days’ Supply – DSP10 = how many days the medication prescribed should last
- Date Written – DSP03 = Date the DVM ordered the Rx be filled
- Date Filled – DSP05 = Date the medication is sent home/out of the hospital/picked up
- Payment Type – DSP16 = Select the option “Patient Paid”
Drug Information
- The National Drug Code (NDC), as defined by the FDA, is “Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs.” The Rx number is assigned to a prescription by the dispensing clinic/pharmacy. IF IT IS A COMPOUNDED DRUG, CALL THE COMPOUNDING PHARMACY TO OBTAIN THE NDC NUMBER.
- – When reporting NDC numbers to the clearinghouse, they must be in a (5)-(4)-(2) format. This means, that the 3-segment number previously described must have 5 digits in the first section, 4 digits in the second section, and 2 digits in the last section. The NDC number can be found printed directly on the bottle of the medication. If any of the 3 sections indicated have less digits than the format requires, you must put a “0” before the number. An example is Gabapentin 100mg Capsules which has an NDC number of 49483-605-50. As we see, the second section only has 3 numbers, and reporting requires it to have 4 numbers. Therefore, when going to report this drug, it would be reported as 49483-0605-50. Entering the “0” before the “605” gives the second section a 4-digit code as required.
- NDC Number – DSP08 = NDC Number of the medication listed in the 5-4-2 format. If the NDC number is less than the 5 digits, 4 digits, or 2 digits, add a zero to the front of the number. (i/e if a drug NDC is 1234-123-1, it should be entered as 01234-0123-01 ENTER THE NDC NUMBER WITHOUT HYPHENS.
- Quantity – DSP09 = quantity of product filled
- Units – DSP11 = drop down and select one: each, milligram, milliliter
- Select the “SAVE” button- follow prompts on next page to confirm submission
- DEA Number – PRE02 = Prescribing DVM DEA Number (This is the DEA number of the DVM who prescribed the drug.) after entering, click the magnifying glass next to the form field and will usually autopopulate the remaining information in this category (usually have to enter rest of information manually the first time, but then will autopopulate thereafter)
- First Name – PRE06 = Prescribing DVM First Name
Last Name – PRE05 = Prescribing DVM Last name
Prescriptions
- Rx Number – DSP02 = Prescription number found on the Rx bottle being sent home
Refill Number – DSP06 = If this is the first time filled, this is 0. If it is the first time being refilled, this is 1, etc.- THIS IS NOT HOW MANY REFILLS ARE AUTHORIZED - Authorized Refills – DSP04 – number of refills allowed
- Days’ Supply – DSP10 = how many days the medication prescribed should last
- Date Written – DSP03 = Date the DVM ordered the Rx be filled
- Date Filled – DSP05 = Date the medication is sent home/out of the hospital/picked up
- Payment Type – DSP16 = Select the option “Patient Paid”
Drug Information
- The National Drug Code (NDC), as defined by the FDA, is “Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs.” The Rx number is assigned to a prescription by the dispensing clinic/pharmacy. IF IT IS A COMPOUNDED DRUG, CALL THE COMPOUNDING PHARMACY TO OBTAIN THE NDC NUMBER.
- – When reporting NDC numbers to the clearinghouse, they must be in a (5)-(4)-(2) format. This means, that the 3-segment number previously described must have 5 digits in the first section, 4 digits in the second section, and 2 digits in the last section. The NDC number can be found printed directly on the bottle of the medication. If any of the 3 sections indicated have less digits than the format requires, you must put a “0” before the number. An example is Gabapentin 100mg Capsules which has an NDC number of 49483-605-50. As we see, the second section only has 3 numbers, and reporting requires it to have 4 numbers. Therefore, when going to report this drug, it would be reported as 49483-0605-50. Entering the “0” before the “605” gives the second section a 4-digit code as required.
- NDC Number – DSP08 = NDC Number of the medication listed in the 5-4-2 format. If the NDC number is less than the 5 digits, 4 digits, or 2 digits, add a zero to the front of the number. (i/e if a drug NDC is 1234-123-1, it should be entered as 01234-0123-01 ENTER THE NDC NUMBER WITHOUT HYPHENS.
- Quantity – DSP09 = quantity of product filled
- Units – DSP11 = drop down and select one: each, milligram, milliliter
- Select the “SAVE” button- follow prompts on next page to confirm submission
- Rx Number – DSP02 = Prescription number found on the Rx bottle being sent home
Refill Number – DSP06 = If this is the first time filled, this is 0. If it is the first time being refilled, this is 1, etc.- THIS IS NOT HOW MANY REFILLS ARE AUTHORIZED - Authorized Refills – DSP04 – number of refills allowed
- Days’ Supply – DSP10 = how many days the medication prescribed should last
- Date Written – DSP03 = Date the DVM ordered the Rx be filled
- Date Filled – DSP05 = Date the medication is sent home/out of the hospital/picked up
- Payment Type – DSP16 = Select the option “Patient Paid”
Drug Information
- The National Drug Code (NDC), as defined by the FDA, is “Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs.” The Rx number is assigned to a prescription by the dispensing clinic/pharmacy. IF IT IS A COMPOUNDED DRUG, CALL THE COMPOUNDING PHARMACY TO OBTAIN THE NDC NUMBER.
- – When reporting NDC numbers to the clearinghouse, they must be in a (5)-(4)-(2) format. This means, that the 3-segment number previously described must have 5 digits in the first section, 4 digits in the second section, and 2 digits in the last section. The NDC number can be found printed directly on the bottle of the medication. If any of the 3 sections indicated have less digits than the format requires, you must put a “0” before the number. An example is Gabapentin 100mg Capsules which has an NDC number of 49483-605-50. As we see, the second section only has 3 numbers, and reporting requires it to have 4 numbers. Therefore, when going to report this drug, it would be reported as 49483-0605-50. Entering the “0” before the “605” gives the second section a 4-digit code as required.
- NDC Number – DSP08 = NDC Number of the medication listed in the 5-4-2 format. If the NDC number is less than the 5 digits, 4 digits, or 2 digits, add a zero to the front of the number. (i/e if a drug NDC is 1234-123-1, it should be entered as 01234-0123-01 ENTER THE NDC NUMBER WITHOUT HYPHENS.
- Quantity – DSP09 = quantity of product filled
- Units – DSP11 = drop down and select one: each, milligram, milliliter
- Select the “SAVE” button- follow prompts on next page to confirm submission
- The National Drug Code (NDC), as defined by the FDA, is “Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs.” The Rx number is assigned to a prescription by the dispensing clinic/pharmacy. IF IT IS A COMPOUNDED DRUG, CALL THE COMPOUNDING PHARMACY TO OBTAIN THE NDC NUMBER.
- – When reporting NDC numbers to the clearinghouse, they must be in a (5)-(4)-(2) format. This means, that the 3-segment number previously described must have 5 digits in the first section, 4 digits in the second section, and 2 digits in the last section. The NDC number can be found printed directly on the bottle of the medication. If any of the 3 sections indicated have less digits than the format requires, you must put a “0” before the number. An example is Gabapentin 100mg Capsules which has an NDC number of 49483-605-50. As we see, the second section only has 3 numbers, and reporting requires it to have 4 numbers. Therefore, when going to report this drug, it would be reported as 49483-0605-50. Entering the “0” before the “605” gives the second section a 4-digit code as required.
- NDC Number – DSP08 = NDC Number of the medication listed in the 5-4-2 format. If the NDC number is less than the 5 digits, 4 digits, or 2 digits, add a zero to the front of the number. (i/e if a drug NDC is 1234-123-1, it should be entered as 01234-0123-01 ENTER THE NDC NUMBER WITHOUT HYPHENS.
- Quantity – DSP09 = quantity of product filled
- Units – DSP11 = drop down and select one: each, milligram, milliliter
- Select the “SAVE” button- follow prompts on next page to confirm submission